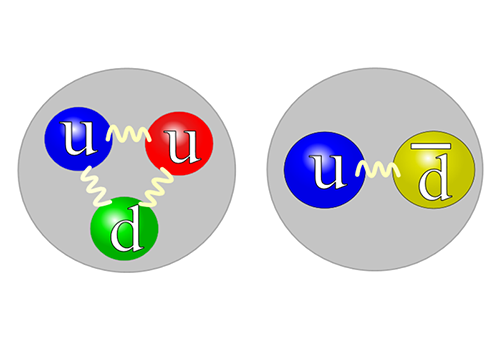
When the universe was created, strong magnetic fields existed. The same type of magnetic fields can be created in experiments when heavy ions are made to collide. We study how elementary particles behave in these fields. The theoretical understanding of this is important in order to explain the experiments as well the history of the …
Fortsett å lese «Understanding fundamental particles in strong magnetic fields»